The Oncology Therapeutics and Drug Development is a multidisciplinary, peer-reviewed platform dedicated to advancing research in cancer treatment innovation and pharmaceutical development. The journal focuses on the discovery, optimization, and clinical evaluation of novel anticancer agents including small molecules, biologics, targeted therapies, immunotherapies, and combination strategies.
Its scope encompasses the full spectrum of oncology drug development, from preclinical mechanistic studies and translational research to clinical trial outcomes, biomarker discovery, and therapeutic optimization. The journal also highlights emerging technologies such as nanomedicine, precision oncology, AI-driven drug design, and advanced delivery systems aimed at improving therapeutic index and patient outcomes.
By bringing together researchers, clinicians, pharmacologists, and industry professionals, the Oncology Therapeutics and Drug Development serves as a comprehensive resource for cutting-edge advancements shaping the future of cancer therapy. The journal is committed to fostering innovation, reducing the burden of cancer, and accelerating the development of safer, more effective oncology therapeutics.
The Oncology Therapeutics and Drug Development is a peer-reviewed, open-access journal committed to advancing scientific research and innovation in the development of anticancer therapies. The journal serves as an international platform for the publication of high-quality research, breakthrough discoveries, and emerging therapeutic approaches that contribute to improved cancer treatment, drug discovery, and clinical outcomes.
The journal covers a comprehensive range of topics related to oncology therapeutics and drug development, including but not limited to:
Cancer Biology & Molecular Oncology
- Tumor biology, signaling pathways, oncogenes, tumor suppressors, and microenvironment studies
- Molecular mechanisms of cancer progression, metastasis, and invasion
- Biomarker discovery, prognostic indicators, and molecular diagnostics
Targeted Therapies & Precision Oncology
- Development and evaluation of small molecules, monoclonal antibodies, and peptide-based therapies
- Precision medicine approaches, genetic and molecular profiling for individualized treatment
- Resistance mechanisms and strategies to overcome therapeutic resistance
Immuno-Oncology & Cell-Based Therapies
- Immune checkpoint inhibitors, cancer vaccines, and adoptive cell therapies (CAR-T, TILs)
- Tumor-immune interactions, immune modulation, and microenvironment targeting
- Combination immunotherapies and translational studies
Drug Discovery & Preclinical Development
- High-throughput screening, lead optimization, and structure-based drug design
- Pharmacokinetics, pharmacodynamics, and preclinical efficacy evaluation
- In vitro and in vivo models for cancer therapeutics development
Clinical Trials & Translational Research
- Early-phase and late-phase clinical trials in oncology
- Biomarker-driven patient stratification and trial design
- Safety, efficacy, and outcome measures in therapeutic evaluation
Nanomedicine & Advanced Drug Delivery in Oncology
- Nanoparticle-based drug carriers, liposomes, and polymeric systems
- Targeted delivery, controlled release, and tumor-specific therapeutics
- Toxicity, biocompatibility, and translational nanomedicine studies
Computational Oncology & AI in Drug Development
- In silico modeling, molecular docking, and drug-target interaction simulations
- AI/ML-based predictive models for drug efficacy and toxicity
- Systems biology approaches for personalized therapy design
Emerging Therapies & Translational Innovations
- Novel therapeutic modalities such as epigenetic drugs, RNA therapeutics, and oncolytic viruses
- Combination strategies integrating chemotherapy, immunotherapy, and targeted agents
- Regulatory, ethical, and commercialization considerations in oncology drug development
Cancer Biology & Molecular Mechanisms
- Oncogenic signaling pathways, tumor suppressor networks, and epigenetic regulation
- Tumor microenvironment, angiogenesis, hypoxia, and stromal interactions
- Cancer stem cells, epithelial-to-mesenchymal transition (EMT), and metastasis
- Molecular biomarkers for diagnosis, prognosis, and therapeutic response
Targeted Therapies & Precision Oncology
- Small molecule inhibitors, monoclonal antibodies, and receptor-targeted drugs
- Gene- and pathway-specific therapy development
- Overcoming drug resistance, tumor heterogeneity, and adaptive therapy strategies
- Companion diagnostics and precision medicine approaches
Immuno-Oncology & Advanced Cellular Therapies
- Immune checkpoint blockade (PD-1, PD-L1, CTLA-4) and immune modulation
- Adoptive cell therapies (CAR-T, TCR, NK cells) and tumor-infiltrating lymphocytes (TILs)
- Cancer vaccines, cytokine therapies, and combination immunotherapies
- Tumor-immune microenvironment studies and immunotherapy biomarkers
Drug Discovery, Preclinical & Translational Research
- High-throughput drug screening, combinatorial therapy testing, and lead optimization
- Pharmacokinetics, pharmacodynamics, toxicology, and preclinical efficacy evaluation
- In vitro 3D tumor models, organoids, patient-derived xenografts (PDX)
- Translational studies bridging lab findings to clinical trials
Clinical Development & Trials in Oncology
- Phase I–IV clinical trials, trial design, endpoints, and patient stratification
- Biomarker-driven and adaptive clinical trial methodologies
- Evaluation of safety, efficacy, and treatment outcomes
- Regulatory frameworks, ethical considerations, and clinical trial reporting
Nanomedicine & Targeted Drug Delivery Systems
- Nanoparticle-based therapeutics, liposomes, micelles, and dendrimers
- Tumor-targeted delivery, controlled release, and pharmacological optimization
- Theranostics combining diagnostics and therapeutics at the nanoscale
- Toxicity assessment, biocompatibility, and translational nanomedicine research
Gene & RNA-Based Therapeutics
- RNA therapeutics, including siRNA, miRNA, mRNA-based treatments
- CRISPR/Cas gene editing and genome-targeted oncology therapies
- Viral vectors, oncolytic viruses, and gene-modified cellular therapies
- Epigenetic modulators and chromatin-targeting drugs
Computational Oncology & Artificial Intelligence Applications
- Drug-target interaction modeling, molecular docking, and predictive toxicology
- AI/ML-based identification of drug candidates and personalized therapy prediction
- Systems biology and network pharmacology in cancer therapy design
- Big data analytics for patient stratification and clinical outcome prediction
Emerging Therapies & Translational Innovations
- Combination therapies integrating chemotherapy, targeted therapy, and immunotherapy
- Novel small molecules, peptides, and biologics for hard-to-treat cancers
- Organoids, lab-on-chip, and personalized tumor models for therapy testing
- Policy, regulatory, and ethical considerations in oncology drug development
The journal publishes original research articles, reviews, short communications, case studies, clinical trial reports, and expert perspectives that meaningfully advance the understanding and development of innovative cancer therapeutics.
The Oncology Therapeutics and Drug Development aims to foster collaboration among oncologists, pharmaceutical scientists, researchers, clinicians, industry experts, and policymakers to accelerate the discovery and delivery of effective cancer treatments worldwide. By promoting scientific excellence and knowledge exchange, the journal strives to support the global effort to reduce the burden of cancer through enhanced drug development and therapeutic innovation.
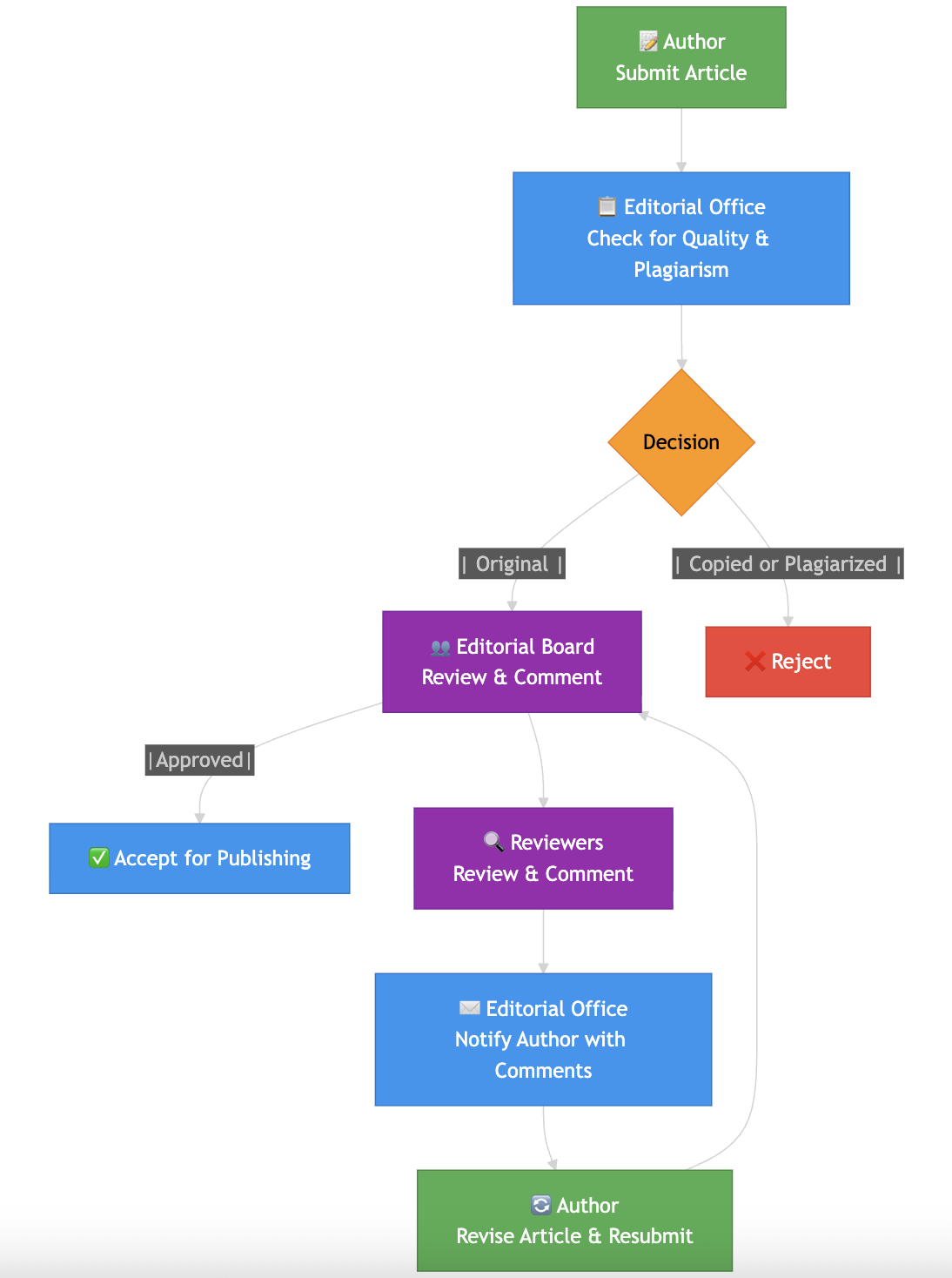
Editorial and Peer-Review Policy
Oncology Therapeutics and Drug Development adheres to a strict single-blind peer-review process to maintain the quality, integrity,
and scientific validity of all published work.
Key features include:
-
Each manuscript is reviewed by independent experts in the relevant field, along with oversight from an academic editor.
-
Reviewers can see author details, but the identities of the reviewers remain hidden from the authors.
-
The evaluations emphasize scientific merit, originality, methodological rigor, ethical
compliance, and how well the work fits within the journal's scope.
-
Every submission goes through plagiarism checks and ethical compliance assessments
before the review process begins.
-
The final decision on publication is based on the reviewer's recommendations and the
editor's evaluation.
Publication Frequency & Format
This journal comes out Bi-Annual (Two issues per year). Accepted articles are
made available online in both HTML and PDF formats, ensuring that scholarly work is accessible
and disseminated promptly.
Open Access Policy
Oncology Therapeutics and Drug Development operates under a fully open access model. This means all published content is freely
accessible to readers around the globe, without any subscription or paywall. Authors maintain
the copyright to their work, and the content is shared under an appropriate open-access license.
Indexing & Archiving
Oncology Therapeutics and Drug Development is dedicated to upholding high standards with the goal of being included in reputable
scientific indexing and abstracting services. The journal also ensures long-term digital
preservation through recognized archives and repositories, guaranteeing permanent access and
discoverability.
Manuscript Submission
Authors are encouraged to submit their manuscripts through the journal's Online Submission
System (Submit Manuscript) or by emailing them directly to the
Editorial Office at
support@globalmeetx.com.
Manuscripts must adhere to the journal's author guidelines regarding structure, formatting,
referencing style, ethical approvals, and necessary disclosures (such as conflicts of interest
and funding).
Publication Ethics & Integrity
Oncology Therapeutics and Drug Development follows internationally recognized standards for publication ethics and research integrity,
which include (but are not limited to):
- Make sure you have ethical approval.
- Ensure informed consent and guarantee confidentiality.
- Declare any conflicts of interest.
- Be transparent about data availability.
- Clarify authorship criteria and the roles of contributors.
- Conduct anti-plagiarism checks.
- Have a clear policy for retraction and correction.