About the Journal
Overview
The Journal of Drug Discovery and Research is a peer-reviewed, open-access publication dedicated to advancing scientific knowledge in all areas of drug development and therapeutic innovation. The journal serves as a dynamic platform for researchers, scientists, and healthcare professionals to share original research articles, comprehensive reviews, case studies, and short communications that explore the discovery, design, development, and evaluation of novel drugs.
With a focus on both basic and applied aspects of drug discovery, the journal welcomes contributions across a broad spectrum of disciplines, including medicinal chemistry, pharmacology, toxicology, molecular modeling, high-throughput screening, pharmacokinetics, and drug delivery systems. Emphasizing innovation and translational research, it also highlights studies that bridge the gap between laboratory findings and clinical application.
Our mission is to promote scientific excellence and facilitate the exchange of knowledge that can drive future breakthroughs in drug discovery and therapeutic strategies. By offering timely publication and global visibility, the Journal of Drug Discovery and Research supports the ongoing efforts of the scientific community in improving human health.
Aim and Scope
The Journal of Drug Discovery and Research publishes original research articles, reviews, short communications, case studies, and technical perspectives that explore all facets of drug discovery, pharmaceutical innovation, and therapeutic development. We welcome submissions in a variety of thematic areas, including (but not limited to):
Medicinal Chemistry & Drug Design
- Rational drug design, structure–activity relationships (SAR), and lead optimization
- Synthesis, characterization, and evaluation of novel bioactive compounds
- Natural products, synthetic molecules, and peptide-based therapeutics
- Chemical biology, target identification, and mechanistic studies
Computational & AI-Driven Drug Discovery
- Molecular docking, virtual screening, pharmacophore modeling, and QSAR analyses
- Molecular dynamics simulations and in silico ADMET predictions
- AI/ML-enabled hit identification, de novo drug design, and systems-level modeling
Pharmacology, Toxicology & ADME Studies
- In vitro and in vivo pharmacological evaluations of drug candidates
- Toxicity studies, safety profiling, and dose-response assessments
- Pharmacokinetics (PK), pharmacodynamics (PD), and drug–drug interaction analyses
- Biomarker discovery, target validation, and mechanistic pharmacology
Drug Delivery Systems & Nanopharmaceuticals
- Nanocarriers, liposomes, polymeric nanoparticles, and targeted delivery platforms
- Controlled-release systems, transdermal, mucosal, and inhalation delivery technologies
- Biocompatibility, stability, and performance of advanced delivery systems
Biopharmaceuticals, Biotechnology & Advanced Therapeutics
- Monoclonal antibodies, recombinant proteins, and biosimilars
- Gene therapy, RNA-based therapeutics, and cellular therapies
- Immunotherapy, vaccine development, and engineered biologics
Pharmaceutical Analysis & Quality Assurance
- Analytical method development, validation, and impurity profiling
- Chromatographic, spectroscopic, and mass spectrometric characterization
- Stability studies, quality control, and regulatory compliance
- Analytical approaches for complex and biological formulations
Clinical Research & Translational Drug Development
- Preclinical-to-clinical translation strategies
- Early-phase clinical trials, dose optimization, and therapeutic index assessment
- Precision medicine, personalized therapies, and biomarker-driven clinical studies
- Pharmacovigilance, real-world evidence, and post-marketing evaluations
Natural Products, Traditional Medicines & Bioactive Compounds
- Isolation, characterization, and pharmacological assessment of natural products
- Ethnopharmacology and traditional medicinal systems
- Standardization, quality, and mechanistic insights into natural therapeutics
Emerging, Interdisciplinary & Applied Pharmaceutical Sciences
- Drug repurposing, polypharmacology, and multi-target therapeutics
- Industrial drug development, formulation technologies, and scale-up innovations
- Regulatory science, policy, and ethics in drug research and development
- Intellectual property, commercialization, and technology transfer
Editorial and Peer-Review Policy
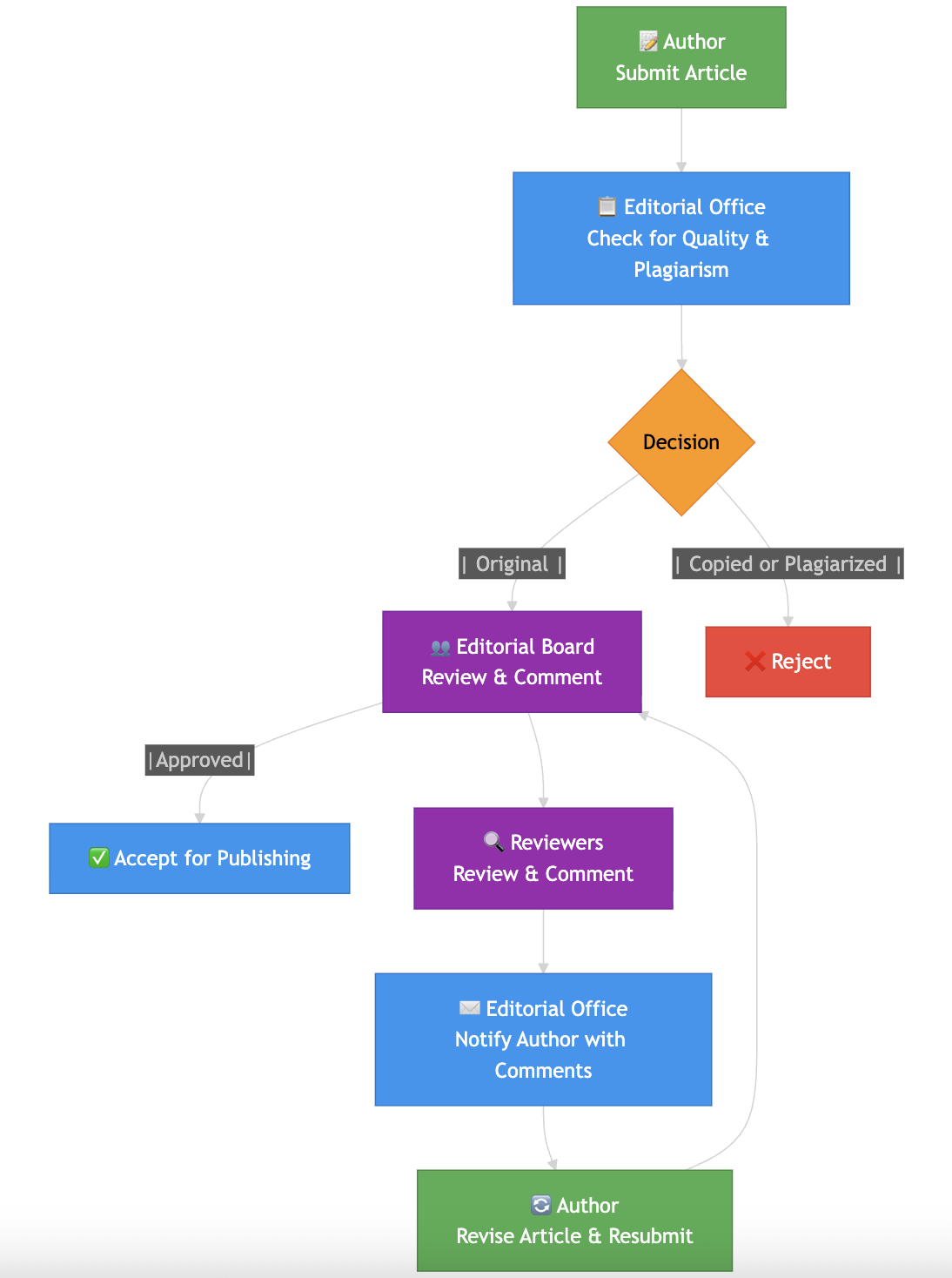
Journal of Drug Discovery and Research adheres to a strict single-blind peer-review process to maintain the quality, integrity,
and scientific validity of all published work.
Key features include:
-
Each manuscript is reviewed by independent experts in the relevant field, along with oversight from an academic editor.
-
Reviewers can see author details, but the identities of the reviewers remain hidden from the authors.
-
The evaluations emphasize scientific merit, originality, methodological rigor, ethical
compliance, and how well the work fits within the journal's scope.
-
Every submission goes through plagiarism checks and ethical compliance assessments
before the review process begins.
-
The final decision on publication is based on the reviewer's recommendations and the
editor's evaluation.
Publication Frequency & Format
This journal comes out Quarterly (Four issues per year). Accepted articles are
made available online in both HTML and PDF formats, ensuring that scholarly work is accessible
and disseminated promptly.
Open Access Policy
Journal of Drug Discovery and Research operates under a fully open access model. This means all published content is freely
accessible to readers around the globe, without any subscription or paywall. Authors maintain
the copyright to their work, and the content is shared under an appropriate open-access license.
Indexing & Archiving
Journal of Drug Discovery and Research is dedicated to upholding high standards with the goal of being included in reputable
scientific indexing and abstracting services. The journal also ensures long-term digital
preservation through recognized archives and repositories, guaranteeing permanent access and
discoverability.
Manuscript Submission
Authors are encouraged to submit their manuscripts through the journal's Online Submission
System (Submit Manuscript) or by emailing them directly to the
Editorial Office at
support@globalmeetx.com.
Manuscripts must adhere to the journal's author guidelines regarding structure, formatting,
referencing style, ethical approvals, and necessary disclosures (such as conflicts of interest
and funding).
Publication Ethics & Integrity
Journal of Drug Discovery and Research follows internationally recognized standards for publication ethics and research integrity,
which include (but are not limited to):
- Make sure you have ethical approval.
- Ensure informed consent and guarantee confidentiality.
- Declare any conflicts of interest.
- Be transparent about data availability.
- Clarify authorship criteria and the roles of contributors.
- Conduct anti-plagiarism checks.
- Have a clear policy for retraction and correction.