ABSTRACT
The increasing use of cross-sectional imaging has resulted in a rising incidence of incidentally detected small renal masses (SRMs), defined as enhancing renal tumors ≤4 cm. Management has evolved from routine surgery to individualized, risk-based strategies.
Methods: A structured narrative review was performed following PRISMA-aligned principles. PubMed/MEDLINE, Scopus, Web of Science, and Google Scholar were searched for studies published between January 2000 and December 2025. Eligible studies included adult patients with SRMs managed by active surveillance (AS), partial nephrectomy (PN), or percutaneous thermal ablation. Thirty studies met inclusion criteria and were synthesized qualitatively.
Results: SRMs generally demonstrate slow growth kinetics, with mean growth rates of approximately 0.25–0.3 cm/year, and the risk of metastatic progression during AS remains low (<3%) in appropriately selected patients. PN provides excellent long- term oncologic control and superior renal function preservation and remains the preferred treatment for surgically fit patients. Thermal ablation offers a minimally invasive alternative with favorable functional outcomes and acceptable oncologic control, although local recurrence rates are slightly higher than with PN.
Conclusions: Management of SRMs should be individualized according to patient comorbidity, tumor characteristics, and life expectancy. AS, thermal ablation, and PN each represent valid treatment strategies, and shared decision-making is essential to optimize outcomes.
Keywords: Small Renal Mass; Active Surveillance; Partial Nephrectomy; Thermal Ablation; Renal Cell Carcinoma
INTRODUCTION
The widespread use of advanced cross-sectional imaging modalities, including computed tomography (CT) and magnetic resonance imaging (MRI), has led to a marked increase in the incidental detection of small renal masses (SRMs). SRMs are commonly defined as enhancing renal cortical tumors measuring ≤4 cm, corresponding to clinical stage T1a [1]. These lesions represent a heterogeneous pathological spectrum that includes benign tumors such as oncocytomas and angiomyolipomas, as well as indolent and aggressive subtypes of renal cell carcinoma (RCC) [2].
Historically, most SRMs were managed surgically, with radical nephrectomy frequently performed based on the presumption that all enhancing renal masses posed a substantial oncologic threat. However, growing evidence indicates that many SRMs have limited malignant potential and slow growth kinetics. This understanding has driven a paradigm shift toward individualized management strategies that consider patient age, comorbid conditions, baseline renal function, and tumor biology [3,4,5,6].
Current evidence-based treatment options for SRMs include active surveillance (AS), percutaneous thermal ablation (TA) techniques such as cryoablation and radiofrequency ablation, and nephron-sparing surgery, most commonly partial nephrectomy (PN) [6,7,8]. Contemporary clinical practice guidelines from the American Urological Association (AUA) and the European Association of Urology (EAU) emphasize shared decision-making and endorse AS, TA, and PN in appropriate clinical scenarios [9,10]. Partial nephrectomy remains the preferred intervention for surgically fit patients when technically feasible, while AS and ablation are recommended for patients with limited life expectancy, significant comorbidities, or tumors demonstrating indolent behavior [10,11].
This narrative review summarizes current evidence regarding the natural history of SRMs and compares outcomes associated with AS, TA, and PN.
MATERIALS AND METHODS
This narrative review was conducted using a structured methodology to identify and synthesize evidence related to the management of SRMs (≤4 cm) using AS, PN, and percutaneous TA.
Search Strategy
A comprehensive literature search was performed using PubMed/MEDLINE, Scopus, Web of Science, and Google Scholar, covering studies published between January 2000 and December 2025. Search terms included combinations of “small renal mass,” “SRM,” “T1a renal tumor,” “active surveillance,” “partial nephrectomy,” “nephron-sparing surgery,” “radiofrequency ablation,” “cryoablation,” “thermal ablation,” and “renal mass biopsy.” Additional references were identified through manual review of bibliographies from relevant reviews, meta-analyses, and guideline publications.
The search yielded 784 records across all databases. After removal of 224 duplicate articles, 560 studies underwent title and abstract screening. Of these, 500 records were excluded based on predefined criteria, including case reports, small case series, pediatric populations, non-enhancing or cystic renal lesions, or lack of relevant SRM-specific outcomes. 60 articles underwent full-text review and 30 studies met the inclusion criteria and were incorporated into the qualitative synthesis. The study selection process is summarized in the PRISMA flow diagram (Figure 1).
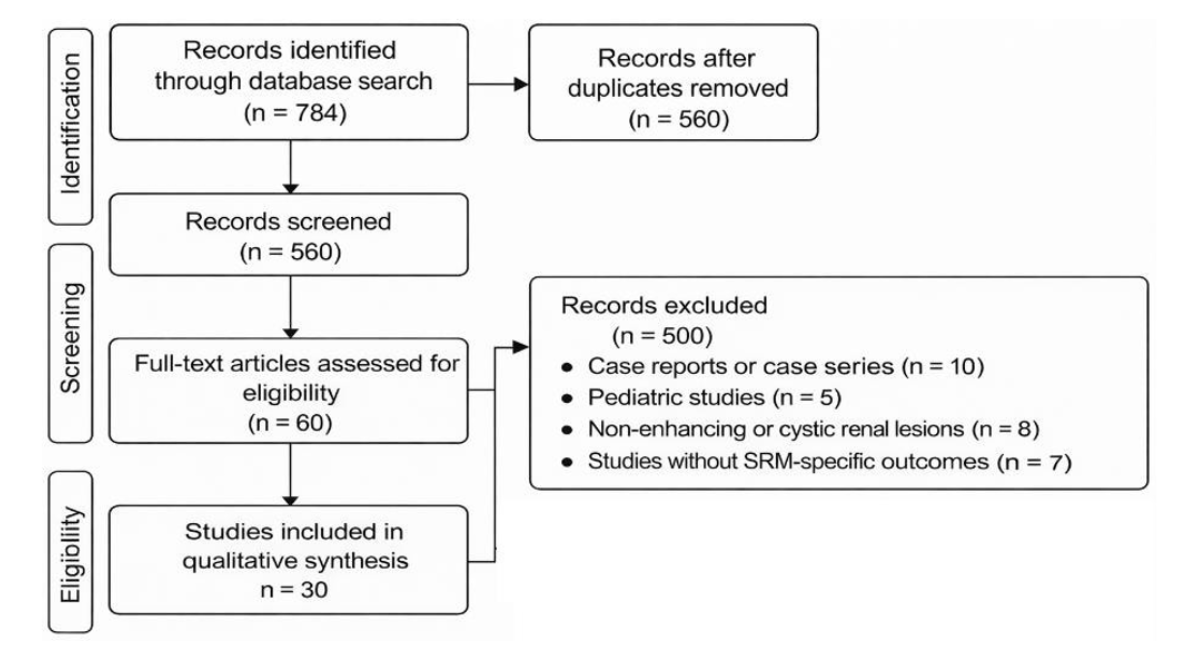
Figure 1 . PRISMA flow diagram of studies selection
Eligibility Criteria
Eligible studies included adult patients (≥18 years) with solid, contrast-enhancing renal masses ≤4 cm (clinical T1a) and reported outcomes related to AS, PN, TA, or renal mass biopsy. Accepted study designs included randomized controlled trials, prospective and retrospective cohort studies, registry analyses, systematic reviews, meta-analyses, and major guideline statements.
Exclusion criteria were case reports or case series involving fewer than 10 patients, pediatric studies, non-enhancing or primarily cystic renal lesions (Bosniak I–III), basic science or animal studies, non-English publications, and studies lacking extractable SRM-specific outcome data.
Study Selection and Data Synthesis
Two reviewers independently screened studies and assessed full texts for eligibility. Disagreements were resolved through discussion or adjudication by a third reviewer. Extracted data included study design, patient demographics, tumor characteristics, treatment modality, follow-up duration, oncologic outcomes, renal function, complications, and recurrence rates. Due to heterogeneity across studies, findings were synthesized qualitatively, with greater emphasis placed on prospective studies, large registries, systematic reviews, and guideline-level evidence.
RESULTS
Renal Mass Biopsy and Clinical Decision-Making
Renal mass biopsy (RMB) has become an increasingly important tool in the evaluation and management of SRMs, particularly when histologic confirmation may influence treatment selection. Contemporary series report diagnostic accuracy rates exceeding 85–90%, with low complication rates and an extremely low risk of tumor seeding (<0.01%) [12,13,14]. RMB is particularly useful in differentiating benign from malignant lesions and identifying indolent RCC subtypes, thereby reducing overtreatment. Several studies demonstrate that biopsy findings alter clinical management in approximately 30–40% of cases, often prompting selection of AS or avoidance of unnecessary intervention [15]. RMB also enhances patient selection for AS and TA by providing histologic and emerging molecular risk stratification, reinforcing its role in personalized SRM management [15,16].
Natural History of Small Renal Masses and Active Surveillance
SRMs generally demonstrate slow growth kinetics, with pooled analyses reporting mean linear growth rates of approximately 0.25–0.3 cm per year [17]. Notably, 20– 30% of SRMs are benign, most commonly angiomyolipoma or oncocytoma [2,4]. Despite concerns regarding malignant potential, the risk of metastatic progression during AS remains consistently low, typically below 2–3%, even with extended follow- up [17,18].
The prospective DISSRM (Delayed Intervention and Surveillance for Small Renal Masses) registry provides strong evidence supporting the safety of AS in well- selected patients. Five-year cancer-specific survival was reported at 100%, with metastasis-free survival of 99% [17]. Other observational cohorts report similarly favorable outcomes, particularly among elderly patients and those with substantial comorbidities [7,18]. Common triggers for delayed intervention include tumor growth exceeding 0.5 cm per year, maximum diameter exceeding 4 cm, or imaging features suggestive of aggressive behavior such as rapid morphologic change or increasing heterogeneity [17,19].
Partial Nephrectomy
Partial nephrectomy is widely regarded as the gold-standard surgical treatment for SRMs in surgically fit patients due to excellent oncologic outcomes and preservation of renal parenchyma [20,21,22,23]. Long-term studies demonstrate cancer-specific survival comparable to radical nephrectomy for T1a tumors, while significantly reducing the risk of chronic kidney disease and associated cardiovascular morbidity [22,23].
The adoption of robotic-assisted PN has further improved perioperative outcomes by reducing complication rates, shortening warm ischemia time, and facilitating postoperative recovery compared with conventional laparoscopic approaches [24,25]. Nevertheless, PN may be technically challenging for centrally located, endophytic, or high-complexity tumors, potentially increasing operative difficulty and perioperative morbidity [26].
Percutaneous Thermal Ablation
Percutaneous thermal ablation, including cryoablation and radiofrequency ablation (RFA), represents a minimally invasive, nephron-sparing option primarily recommended for patients who are poor surgical candidates or those with small, exophytic tumors [6,7,10,27]. Systematic reviews report local tumor control rates of approximately 90–95%. Although these rates are slightly inferior to PN, they are considered oncologically acceptable in appropriately selected patients [28,29]. Evidence suggests that cryoablation may provide lower local recurrence rates than RFA, particularly for tumors larger than 3 cm [28,29].
Advantages of ablation include reduced perioperative morbidity, minimal blood loss, shorter hospital stays, and faster recovery [12,27,28]. However, limitations include higher local recurrence rates compared with PN, challenges in treating centrally located tumors, and limited long-term oncologic data [28,29]. The outcomes of different management strategies are summarized in Table 1 .
|
Management Strategy |
Oncologic Control |
Functional Outcomes |
Perioperative Morbidity |
Typical Candidates |
Key Notes / Limitations |
References |
|
Active Surveillance (AS) |
Excellent for small, indolent tumors; metastatic risk <2–3% |
Preserves renal function |
Minimal |
Elderly, comorbid, small/slow- growing SRMs |
Requires close monitoring; triggers for intervention include growth >0.5 cm/year, size >4 cm, or aggressive imaging features |
|
|
Partial Nephrectomy (PN) |
Gold-standard; long-term cancer-specific survival comparable to radical nephrectomy |
Maximal renal preservation, lowers CKD and CV morbidity |
Moderate; technically complex in central/high- nephrometry- score tumors |
Surgically fit patients; technically feasible tumors |
Robotic or minimally invasive approaches reduce morbidity; perioperative complications possible |
|
|
Thermal Ablation (Cryoablation / RFA) |
Acceptable local control (90–95%); slightly higher local recurrence than PN |
Excellent preservation of renal function |
Low; minimally invasive |
Poor surgical candidates; small, exophytic tumors |
Higher local recurrence than PN; central tumors challenging; long-term data limited; repeat ablation possible |
Table 1: Comparative outcomes of current management strategies for small renal masses (SRMs). This table summarizes the oncologic efficacy, renal functional outcomes, perioperative morbidity, typical patient selection, and key limitations of active surveillance (AS), partial nephrectomy (PN), and thermal ablation (cryoablation or radiofrequency ablation ), based on contemporary evidence and clinical guidelines.
DISCUSSION
Management of SRMs has evolved from routine surgical excision to an individualized, risk-adapted approach informed by tumor biology, patient characteristics, and expected oncologic benefit [6,9,10]. Contemporary evidence indicates that many SRMs, particularly those smaller than 2 cm, demonstrate indolent behavior and low metastatic potential [13,17,18]. Prospective AS cohorts consistently show excellent cancer-specific outcomes with minimal metastatic progression [17,19]. The principal advantage of AS is avoidance of overtreatment and preservation of renal function, particularly important in elderly patients and those with significant comorbidities [7,17,19].
Partial nephrectomy remains the gold-standard intervention for surgically fit patients, offering durable oncologic control while preserving renal function [20-23]. Renal function preservation is increasingly recognized as a determinant of long-term cardiovascular and overall survival, underscoring the importance of nephron-sparing approaches even in patients with normal baseline renal function [22,23]. Nonetheless, PN carries inherent surgical risks and technical challenges, particularly for complex tumors [20,24-26].
Thermal ablation has emerged as an important alternative for patients unfit for surgery [12,27-29]. While local recurrence rates are modestly higher than with PN, excellent renal functional preservation and the possibility of repeat ablation make this approach attractive in selected patients [27,28,29].
Future directions include incorporation of molecular biomarkers, standardization of AS protocols, and expanded comparative effectiveness research. Advances in multiparametric imaging and radiomics may further improve tumor characterization, risk stratification, and individualized treatment selection [5,13,14].
CONCLUSION
The contemporary management of small renal masses has shifted toward a personalized, evidence-based framework that balances oncologic control with preservation of renal function and patient-specific considerations. Active surveillance is supported by robust evidence in carefully selected patients, particularly those with small, indolent tumors or significant comorbidities, demonstrating excellent cancer- specific outcomes and minimal metastatic risk [7,13,17-19]. Partial nephrectomy remains the gold-standard treatment for surgically fit patients, offering durable oncologic control while minimizing the risk of chronic kidney disease and associated cardiovascular morbidity [20-26]. Thermal ablation provides a minimally invasive alternative for patients who are poor surgical candidates, with favorable functional outcomes and acceptable oncologic control, despite slightly higher local recurrence rates [12,27-29]. Renal mass biopsy enhances decision-making by enabling histologic and molecular risk stratification, reducing overtreatment and guiding optimal therapy selection [13,15,16]. Collectively, these strategies reflect a paradigm shift from uniform surgical management to a tailored, patient-centered approach for SRMs.
CONFLICTS OF INTEREST
The authors declare no financial or non-financial conflicts of interest related to this research study
REFERENCES
- Capitanio U, Bedke J, Albiges L, Volpe A, Giles RH, et al. A Renewal of the TNM Staging System for Patients with Renal Cancer To Comply with Current Decision-making: Proposal from the European Association of Urology Guidelines Panel. European Urology. 2022;83(1):3-5.[Crossref] [Google Scholar] [PubMed]
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, et al. Solid renal tumors: an analysis of pathological features related to tumor size. The Journal of urology. 2003;170(6):2217-20.[Crossref] [Google Scholar] [PubMed]
- Finelli A, Ismaila N, Bro B, Durack J, Eggener S, et al. Management of small renal masses: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology. 2017;35(6):668-80.[Crossref] [Google Scholar] [PubMed]
- Sun M, Abdollah F, Bianchi M, et al. Treatment management of small renal masses. Eur Urol. 2012;62:1020–1030. [Crossref] [PubMed]
- Lane BR, Kattan MW . Prognostic models and algorithms in renal cell carcinoma. Urologic Clinics of North America. 2008;35(4):613-25.[Crossref] [Google Scholar] [PubMed]
- Pierorazio PM, Patel HD, Johnson MH, et al. Management of small renal masses: AUA guideline. J Urol. 2016;196:1022–1034. [Crossref]
- Jewett MA, Mattar K, Basiuk J, Morash CG, Pautler SE, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. European urology. 2011;60(1):39-44.[Crossref] [Google Scholar] [PubMed]
- Hora M, Albiges L, Bedke J, Campi R, Capitanio U, et al. European Association of Urology guidelines panel on renal cell carcinoma update on the new World Health Organization classification of kidney tumours 2022: the urologist’s point of view. Eur Urol. 2023;83(2):97-100.[Crossref] [Google Scholar] [PubMed]
- Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, et al. Renal mass and localized renal cancer: AUA guideline. The Journal of urology. 2017;198(3):520-9.[Crossref] [Google Scholar]
- Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. European urology. 2022;82(4):399-410.[Crossref] [Google Scholar] [PubMed]
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. Journal of the National Cancer Institute. 2006;98(18):1331-4.[Crossref] [Google Scholar] [PubMed]
- Atwell TD, et al. Complications following image-guided percutaneous renal ablation. Radiographics. 2012;32:1329–1344. [Crossref]
- Volpe A, Cadeddu JA, Cestari A, Gill IS, Jewett MA, et al. Contemporary management of small renal masses. European urology. 2011;60(3):501-15. Eur Urol. [Crossref] [Google Scholar] [PubMed]
- Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563-77.[Crossref] [Google Scholar] [PubMed]
- Volpe A, Kachura JR, Geddie WR, Evans AJ, Gharajeh A, et al. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. The Journal of urology. 2007;178(2):379-86.[Crossref] [Google Scholar] [PubMed]
- Richard PO, Jewett MA, Bhatt JR, Kachura JR, Evans AJ, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. European urology. 2015;68(6):1007-13.[Crossref] [Google Scholar] [PubMed]
- Patel HD, et al. Growth kinetics and metastasis of renal cell carcinoma in patients managed with active surveillance: results from the DISSRM Registry. J Clin Oncol. 2014;32:2992–2998. [Crossref] [PubMed]
- Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. The Journal of urology. 2006;175(2):425-31. [Crossref] [Google Scholar] [PubMed]
- McIntosh AG, Ristau BT, Ruth K, Jennings R, Ross E, et al. Active surveillance for localized renal masses: tumor growth, delayed intervention rates, and> 5-yr clinical outcomes. European urology. 2018;74(2):157-64.[Crossref] [Google Scholar] [PubMed]
- MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. European urology. 2012;61(5):972-93.[Crossref] [Google Scholar] [PubMed]
- Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. Jama. 2012;307(15):1629-35.[Crossref] [Google Scholar] [PubMed]
- Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. The lancet oncology. 2006;7(9):735-40.[Crossref] [Google Scholar] [PubMed]
- Weight CJ, Lieser G, Larson BT, Gao T, Lane BR, et al. Partial nephrectomy is associated with improved overall survival compared to radical nephrectomy in patients with unanticipated benign renal tumours. European urology. 2010;58(2):293-8.[Crossref] [Google Scholar]
- Khalifeh A, et al. Comparative outcomes of robot-assisted and laparoscopic partial nephrectomy. Eur Urol. 2013;64:731–739. [PubMed]
- Simone G, et al. Long-term oncologic outcomes of minimally invasive partial nephrectomy. Eur Urol. 2018;74:661–671.[PubMed]
- Parsons RB, Canter D, Kutikov A, Uzzo RG. RENAL nephrometry scoring system: the radiologist’s perspective. American Journal of Roentgenology. 2012;199(3):W355-9.. [Crossref] [Google Scholar] [PubMed]
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta‐analysis. Cancer. 2008;113(10):2671-80.[Crossref] [Google Scholar] [PubMed]
- El Dib R, Touma NJ, et al. Cryoablation versus radiofrequency ablation for small renal masses: a systematic review. J Endourol. 2014;28:501–508. [Crossref] [PubMed]
- Georgiades C, Rodriguez R. Efficacy and safety of percutaneous cryoablation for renal cell carcinoma. J Vasc Interv Radiol. 2014;25:867–876. [Crossref] [PubMed]